what orbitals overlap to create the c−h bond in ethene
Multiple Bonds
Past the terminate of this section, you will be able to:
- Describe multiple covalent bonding in terms of atomic orbital overlap
- Relate the concept of resonance to π-bonding and electron delocalization
The hybrid orbital model appears to business relationship well for the geometry of molecules involving single covalent bonds. Is it besides capable of describing molecules containing double and triple bonds? We accept already discussed that multiple bonds consist of σ and π bonds. Next we can consider how we visualize these components and how they chronicle to hybrid orbitals. The Lewis structure of ethene, C2H4, shows us that each carbon atom is surrounded by one other carbon cantlet and two hydrogen atoms.
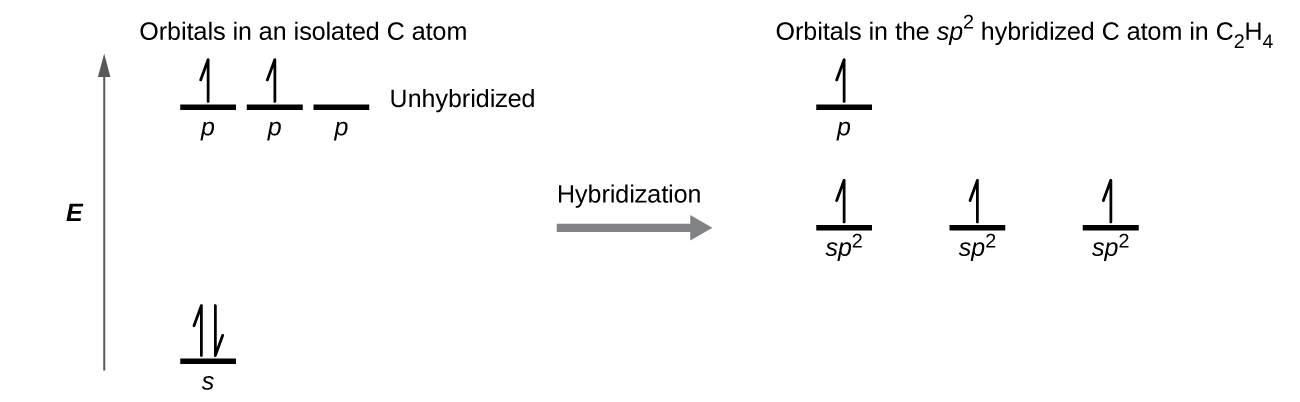
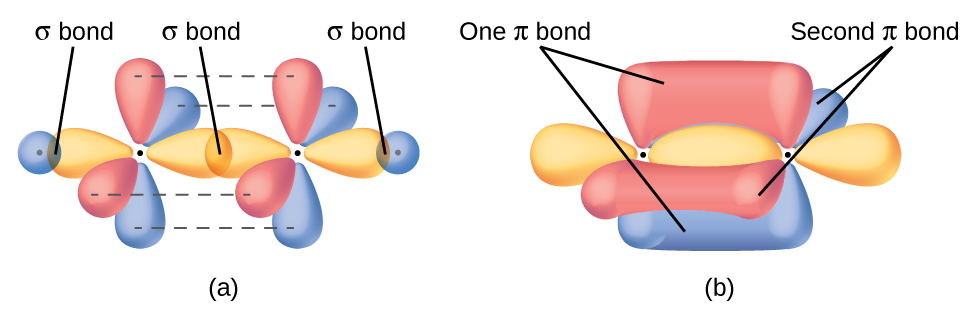
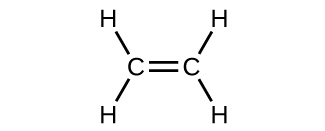 The three bonding regions form a trigonal planar electron-pair geometry. Thus we expect the σ bonds from each carbon atom are formed using a set of sp 2 hybrid orbitals that outcome from hybridization of ii of the iip orbitals and the twos orbital ([link]). These orbitals form the C–H single bonds and the σ bond in the
The three bonding regions form a trigonal planar electron-pair geometry. Thus we expect the σ bonds from each carbon atom are formed using a set of sp 2 hybrid orbitals that outcome from hybridization of ii of the iip orbitals and the twos orbital ([link]). These orbitals form the C–H single bonds and the σ bond in the
double bail ([link]). The π bond in the
double bond results from the overlap of the third (remaining) 2p orbital on each carbon cantlet that is not involved in hybridization. This unhybridized p orbital (lobes shown in red and blue in [link]) is perpendicular to the airplane of the sp 2 hybrid orbitals. Thus the unhybridized iip orbitals overlap in a side-by-side fashion, above and beneath the internuclear axis ([link]) and form a π bond.
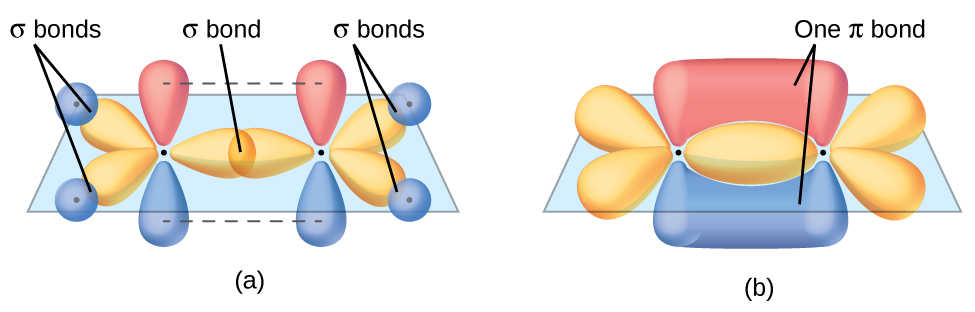
In an ethene molecule, the iv hydrogen atoms and the two carbon atoms are all in the same airplane. If the 2 planes of sp 2 hybrid orbitals tilted relative to each other, the p orbitals would not be oriented to overlap efficiently to create the π bond. The planar configuration for the ethene molecule occurs because information technology is the nigh stable bonding arrangement. This is a meaning difference betwixt σ and π bonds; rotation effectually single (σ) bonds occurs easily because the end-to-cease orbital overlap does not depend on the relative orientation of the orbitals on each atom in the bail. In other words, rotation around the internuclear axis does not alter the extent to which the σ bonding orbitals overlap because the bonding electron density is symmetric about the axis. Rotation virtually the internuclear axis is much more difficult for multiple bonds; however, this would drastically change the off-axis overlap of the π bonding orbitals, essentially breaking the π bail.
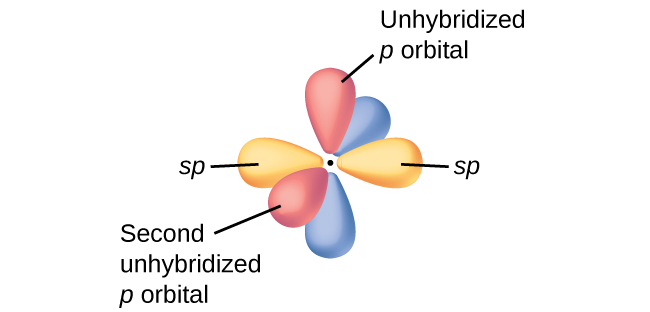
In molecules with sp hybrid orbitals, ii unhybridized p orbitals remain on the atom ([link]). We find this situation in acetylene,
which is a linear molecule. The sp hybrid orbitals of the two carbon atoms overlap end to terminate to form a σ bond between the carbon atoms ([link]). The remaining sp orbitals class σ bonds with hydrogen atoms. The two unhybridized p orbitals per carbon are positioned such that they overlap side past side and, hence, form two π bonds. The 2 carbon atoms of acetylene are thus bound together by one σ bond and two π bonds, giving a triple bond.
Hybridization involves only σ bonds, lone pairs of electrons, and unmarried unpaired electrons (radicals). Structures that business relationship for these features describe the correct hybridization of the atoms. However, many structures also include resonance forms. Think that resonance forms occur when various arrangements of π bonds are possible. Since the arrangement of π bonds involves just the unhybridized orbitals, resonance does non influence the assignment of hybridization.
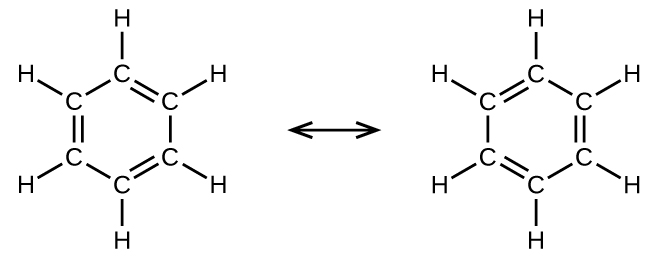
For example, molecule benzene has two resonance forms ([link]). We tin can use either of these forms to determine that each of the carbon atoms is bonded to three other atoms with no lone pairs, and so the correct hybridization is sp 2. The electrons in the unhybridized p orbitals course π bonds. Neither resonance structure completely describes the electrons in the π bonds. They are not located in 1 position or the other, but in reality are delocalized throughout the ring. Valence bail theory does not easily accost delocalization. Bonding in molecules with resonance forms is ameliorate described by molecular orbital theory. (Run into the next module.)
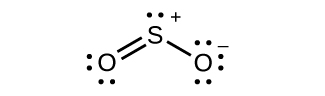
Assignment of Hybridization Involving Resonance Some acid rain results from the reaction of sulfur dioxide with atmospheric h2o vapor, followed by the formation of sulfuric acrid. Sulfur dioxide, SO2, is a major component of volcanic gases as well as a production of the combustion of sulfur-containing coal. What is the hybridization of the S atom in Thentwo?
Solution The resonance structures of Then2 are
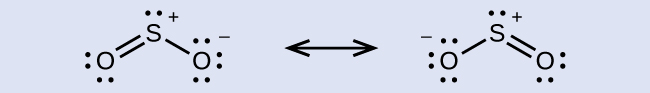 The sulfur atom is surrounded by two bonds and one lone pair of electrons in either resonance structure. Therefore, the electron-pair geometry is trigonal planar, and the hybridization of the sulfur atom is sp 2.
The sulfur atom is surrounded by two bonds and one lone pair of electrons in either resonance structure. Therefore, the electron-pair geometry is trigonal planar, and the hybridization of the sulfur atom is sp 2.
Check Your Learning Another acid in acid rain is nitric acid, HNO3, which is produced by the reaction of nitrogen dioxide, NO2, with atmospheric water vapor. What is the hybridization of the nitrogen atom in NOii? (Note: the lone electron on nitrogen occupies a hybridized orbital just as a lone pair would.)
Key Concepts and Summary
Multiple bonds consist of a σ bond located along the axis betwixt two atoms and one or two π bonds. The σ bonds are usually formed by the overlap of hybridized atomic orbitals, while the π bonds are formed by the side-by-side overlap of unhybridized orbitals. Resonance occurs when there are multiple unhybridized orbitals with the appropriate alignment to overlap, so the placement of π bonds can vary.
Chemistry End of Chapter Exercises
The bond free energy of a C–C single bond averages 347 kJ mol−one; that of a
triple bond averages 839 kJ mol−i. Explain why the triple bond is not iii times equally strong as a unmarried bail.
A triple bond consists of one σ bond and ii π bonds. A σ bail is stronger than a π bond due to greater overlap.
For the carbonate ion,
depict all of the resonance structures. Identify which orbitals overlap to create each bond.
A useful solvent that will dissolve salts besides equally organic compounds is the chemical compound acetonitrile, H3CCN. It is nowadays in paint strippers.
(a) Write the Lewis structure for acetonitrile, and indicate the management of the dipole moment in the molecule.
(b) Identify the hybrid orbitals used by the carbon atoms in the molecule to course σ bonds.
(c) Describe the atomic orbitals that form the π bonds in the molecule. Note that it is not necessary to hybridize the nitrogen atom.
(a)* * *
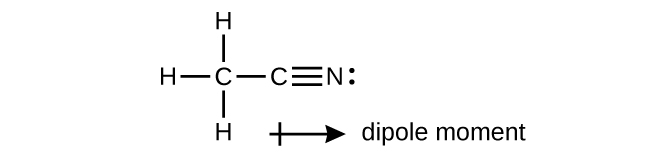
(b) The terminal carbon atom uses sp 3 hybrid orbitals, while the central carbon atom is sp hybridized. (c) Each of the two π bonds is formed by overlap of a 2p orbital on carbon and a nitrogen 2p orbital.
For the molecule allene,
give the hybridization of each carbon cantlet. Will the hydrogen atoms be in the aforementioned plane or perpendicular planes?
Place the hybridization of the key atom in each of the post-obit molecules and ions that incorporate multiple bonds:
(a) ClNO (Northward is the key atom)
(b) CS2
(c) CltwoCO (C is the central atom)
(d) Cl2And so (South is the central atom)
(e) SO2F2 (Southward is the central atom)
(f) XeO2F2 (Xe is the primal atom)
(g)
(Cl is the primal cantlet)
(a) sp two; (b) sp; (c) sp 2; (d) sp three; (e) sp 3; (f) sp 3 d; (m) sp 3
Describe the molecular geometry and hybridization of the N, P, or Due south atoms in each of the post-obit compounds.
(a) H3POfour, phosphoric acid, used in cola soft drinks
(b) NH4NO3, ammonium nitrate, a fertilizer and explosive
(c) S2Cl2, disulfur dichloride, used in vulcanizing rubber
(d) Kfour[O3POPO3], potassium pyrophosphate, an ingredient in some toothpastes
For each of the following molecules, indicate the hybridization requested and whether or not the electrons will be delocalized:
(a) ozone (O3) central O hybridization
(b) carbon dioxide (CO2) fundamental C hybridization
(c) nitrogen dioxide (NO2) central N hybridization
(d) phosphate ion
fundamental P hybridization
(a) sp 2, delocalized; (b) sp, localized; (c) sp 2, delocalized; (d) sp iii, delocalized
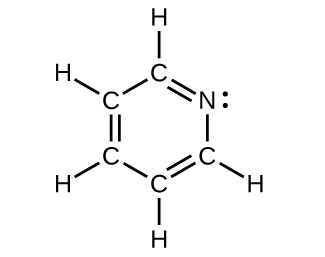
For each of the post-obit structures, determine the hybridization requested and whether the electrons will exist delocalized:
(a) Hybridization of each carbon
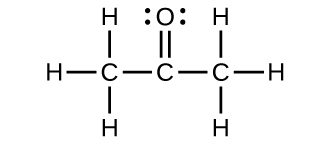 (b) Hybridization of sulfur
(b) Hybridization of sulfur
 (c) All atoms
(c) All atoms
Draw the orbital diagram for carbon in CO2 showing how many carbon atom electrons are in each orbital.
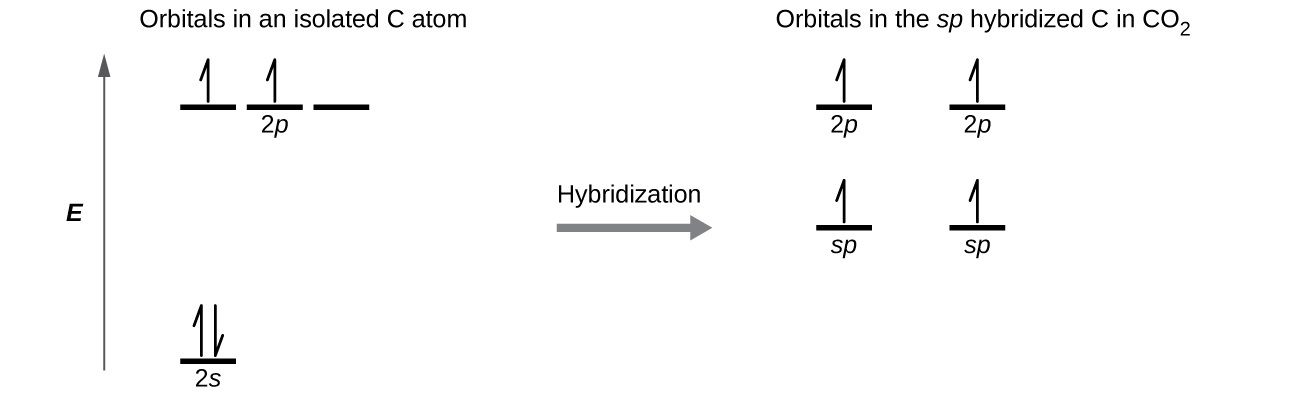 Each of the four electrons is in a dissever orbital and overlaps with an electron on an oxygen atom.
Each of the four electrons is in a dissever orbital and overlaps with an electron on an oxygen atom.

This piece of work is licensed under a Artistic Commons Attribution iv.0 International License.
You can also download for costless at http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@12.1
Attribution:
- For questions regarding this license, delight contact partners@openstaxcollege.org.
- If you use this textbook equally a bibliographic reference, then you should cite information technology equally follows: OpenStax College, Chemistry. OpenStax CNX. http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@12.1.
- If you redistribute this textbook in a print format, then you must include on every physical page the following attribution: "Download for complimentary at http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@12.1."
- If you redistribute part of this textbook, then you lot must retain in every digital format page view (including but non express to EPUB, PDF, and HTML) and on every physical printed page the following attribution: "Download for complimentary at http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@12.1."
Source: https://philschatz.com/chemistry-book/contents/m51058.html
0 Response to "what orbitals overlap to create the c−h bond in ethene"
Post a Comment